News
Keep up to date with everything going on at Milton Park. We cover innovation community news, occupier announcements and Park developments, as well as broader travel and updates.

Quantum cool to fusion fuel
How temperature extremes fuel innovation at Milton Park Home to over 270 organisations and more than 9,000 employees, did you know that Milton Park’s innovation community has (quite literally) some of the...
Search by
-

Where life and work connect
-
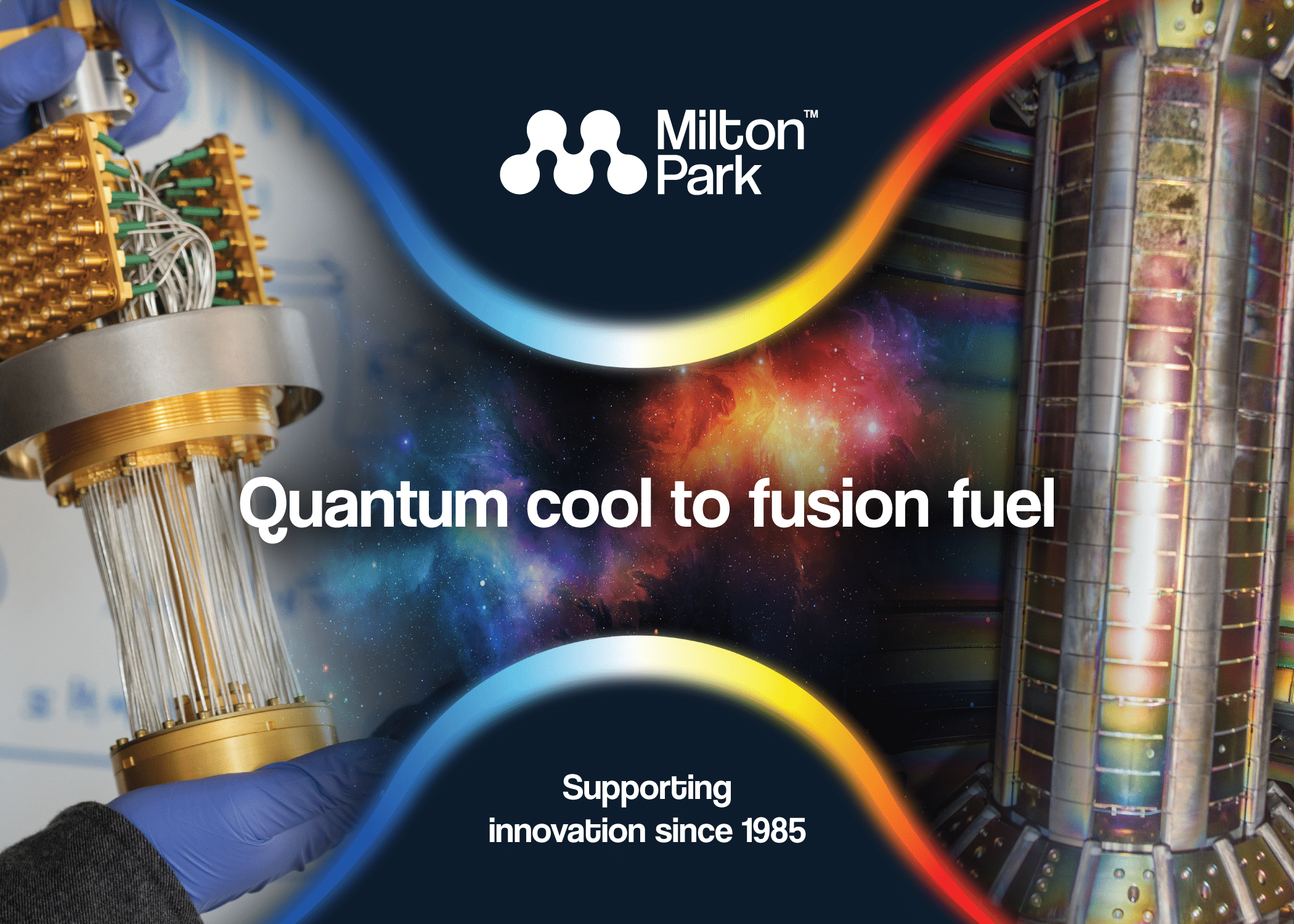
Quantum cool to fusion fuel
-

Wild orchids at the Park
-

Innovation community news – June
-

Eateries and amenities coming to Signal Yard
-

Exploring Milton Park just got easier
-

Park People: Simon Davie
-

Progress continues at Signal Yard
-

A string of award shortlists
-

Exciting rewards for greener travel
-
Innovation community news – May
-

Park People: David Price, Vertex Pharmaceuticals
-

Championing sustainability with Chris Hines MBE
-

Nearly full: Bee House is the place to ‘bee’
-

Innovation community news – April
-
Company Spotlight: TreQ
-

Marking VE Day’s 80th anniversary
-

Signal Yard picking up speed
-

Sustainable initiatives to put a spring in your step
-

Introducing Milton Spark*
-

Introducing Campbell’s Column
-

Park People: Simon Canter, Evotec
-

The future of workspace
-

Didcot Powerhouse shares community ambition for 2032
-

Innovation community news – March
-

Company Spotlight: Bonhams|Cars Online
-
FDA and NICE back Milton Park innovators
-

Pioneering planning scheme shortlisted for economic growth award
-

Momentum Bioscience moves to develop sepsis tests
-

Tetra Tech extends 30-year occupancy
-

Olly Glover MP launches Nebula development at Milton Park
-

Rocket fuelled coffee
-

Celebrating two years of inspiring future innovators
-

Innovation community news – February
-

Park Apprentices: nurturing talent
-

TreQ takes quantum leap with Milton Park move
-

Park People: Dr Annelise Vuidepot, Immunocore
-

Leading nuclear firm relocates to Milton Park
-

Lord Vallance welcomes completion of Nebula development
-

Life Science roundtable with The Business Magazine
-

Company Spotlight: Planted Plates
-

Full steam ahead at Signal Yard
-

New fleet of Park-branded buses improve commutes
-

Park People: Dr David Kingham, Tokamak Energy
-
Innovation community news – January
-

Top trends in life sciences in 2025
-

Simplified ten-day planning to power greener growth at Milton Park
-

Battery technology specialists Agratas joins Milton Park
-

Wrapping up 2024
-

Future Infrastructure Forum
-

Change is coming along the tracks
-

First patient treated with Adaptimmune’s TECELRA(R) (afamitresgene autoleucel)
-

Park People: Meet the Milton Park ‘Elves’
-

Innovation community news – December
-

Company Spotlight: Dress’d
-

A new chapter for Hachette following expansion
-

2024 Travel Survey Update
-

MEPC appoints Curo Construction to deliver Signal Yard
-

Company Spotlight: NeoVac
-

Park People: Dr Peter Sargent, Physiomics
-

First UK facility for New England Biolabs
-
Innovation community news – November
-

Didcot Powerhouse announces 2025 grants theme
-

An update on landscaping at Signal Yard
-

New occupiers create hive of activity at Bee House
-

Company Spotlight: ITGL
-

Amending your amenities
-

What’s in the ‘new backyard’?
-

Innovation community news – October
-

Milton Park scoops national award for green innovation
-

Park People: Dr Yubiao Niu, Nium
-

Timelapse shows construction progress on Nebula development
-

From tokamaks to treatments for diseases
-

Company Spotlight: Immunocore
-

Joining the dots at Signal Yard
-

Occupiers announced as OBN Awards 2024 Finalists
-

Park People: Adam Stoten, Evotec
-
Innovation community news – September
-

Travel Survey 2024
-

Milton Park biodiversity update
-

Signalling change at Park Centre
-

Innovation community news – August
-

Park People: Poppy Roworth, Arctoris
-

Amenities update – a relocation, a revamp and a retirement
-
Innovation community news – July
-

Keeping you posted
-

Company Spotlight: Evotec
-

Park People: George Soanes, LTi Metaltech
-

Celebrating one hundred years of human endeavours at Milton Park
-

From Spitfire wings to spinouts
-

Company Spotlight: ASA Landscape Architects
-

Park People: Abdirahman Hassan, Apollo Pharmacy
-

Innovation community news – June
-

Pharmacy moving
-
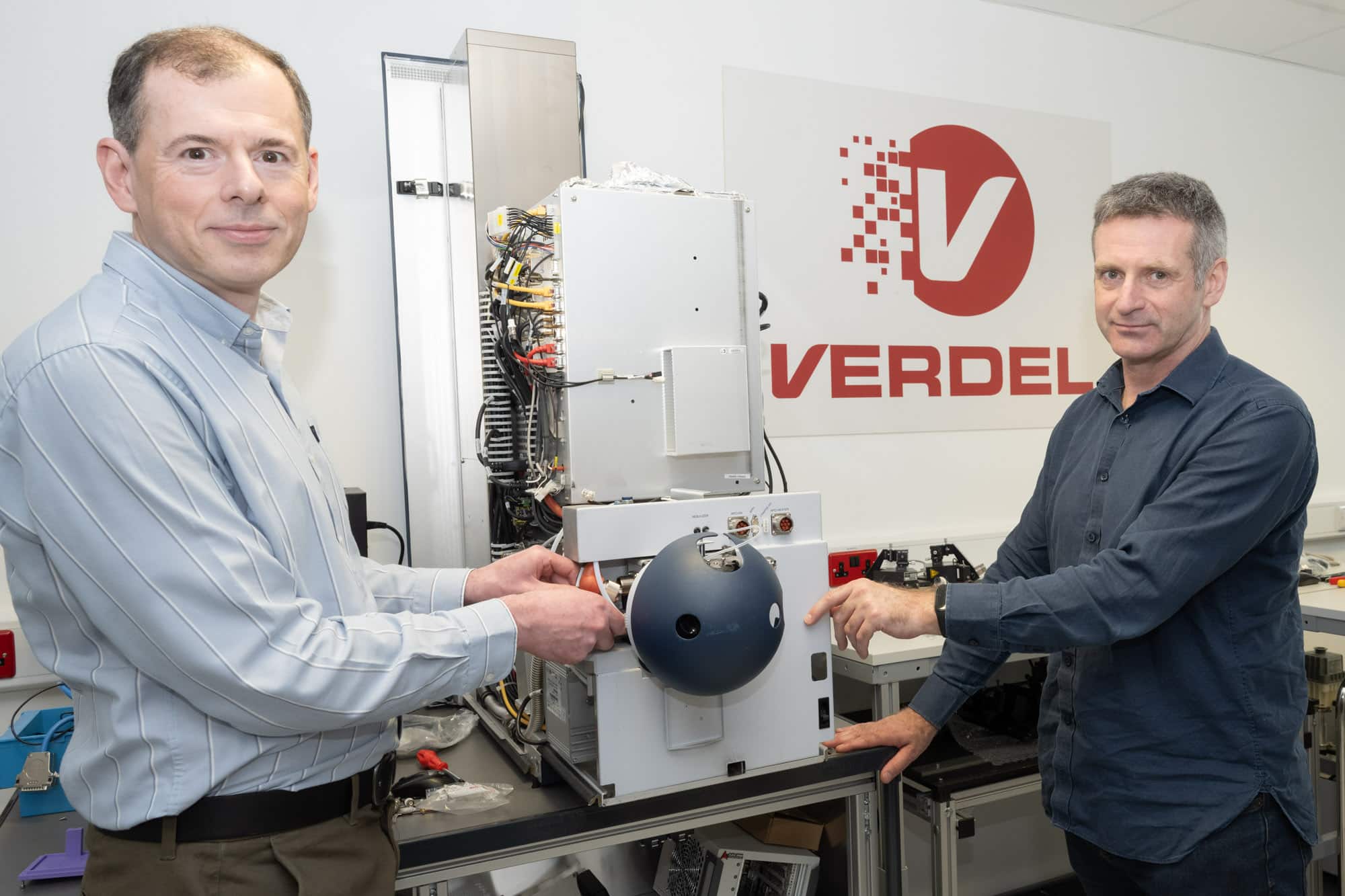
Verdel relocates to develop mass spectrometry at Milton Park
-

Work Experience at Milton Park
-

Nebula’s sustainable timber beams rise out of the ground
-

Park People: Augusto Bartolome, CEO at Electrogenos
-

Company Spotlight: Whittard of Chelsea
-

Fitted labs give boost to vaccine biotech NeoVac
-

Dress’d to impress
-

Low Mow May
-

Park People: Paul Dunning, Horsebox Coffee
-

Company Spotlight: Nium
-

Milton Park’s new bus names
-

Jisc moves to Milton Park
-

Powerhouse grant recipients announced
-

Milton Park companies transforming healthcare
-

The future of AV buses
-

Park occupiers have a ‘spring in their step’
-

Schools programme “incredibly well received” at UKSPA conference
-

Flying visit to Nebula development
-

Park People: Elin Cunningham, Oxitec
-

Powerhouse launches 2023 Impact Report
-

Company Spotlight: Electrogenos
-

Recycled garden
-
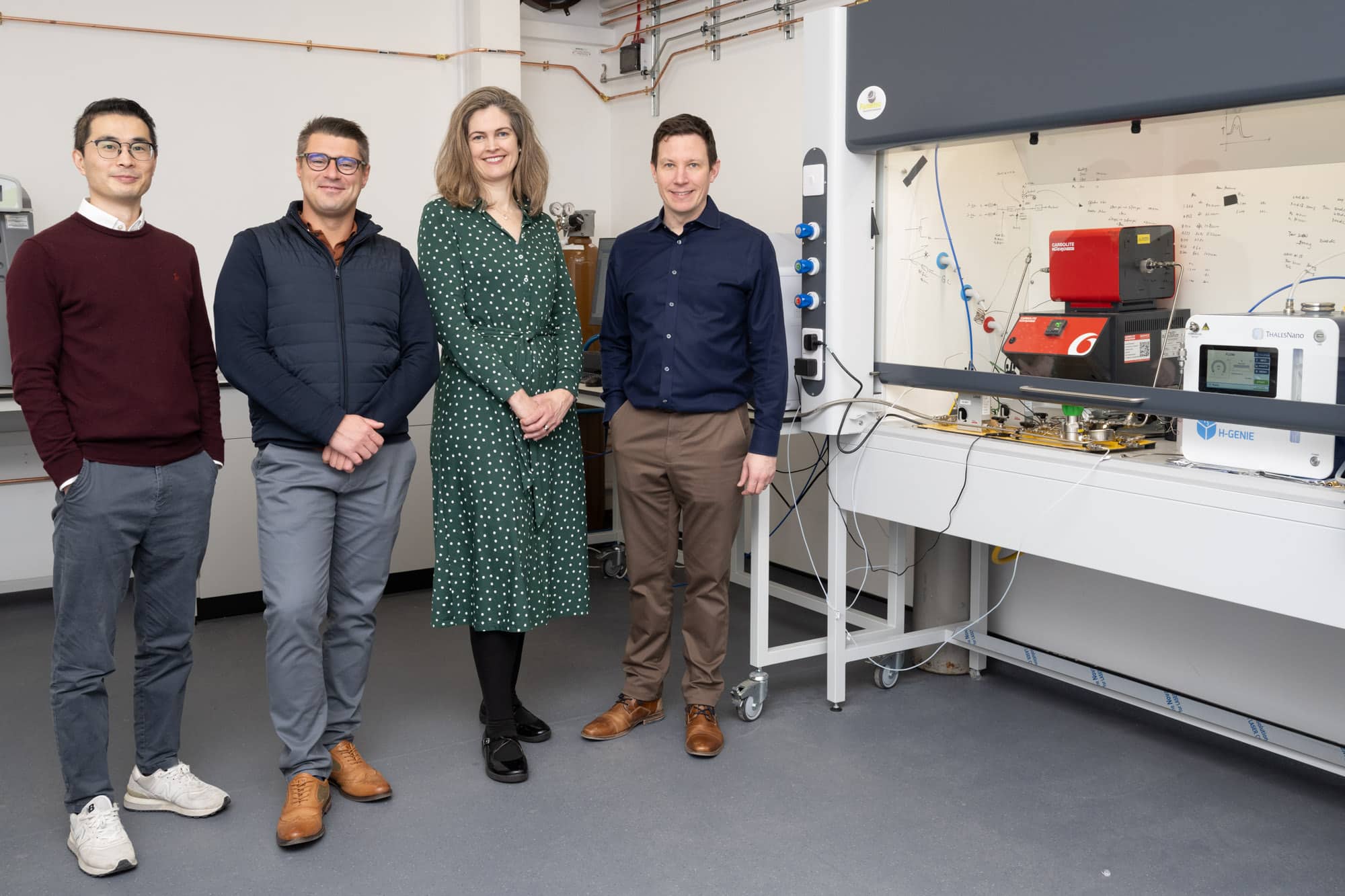
Green-tech company relocates R&D to Milton Park
-

Milton Park inspires 1,000 students to explore STEAM-powered careers
-

Scientific advances at Milton Park
-

Monthly prizes for car sharers
-

Investment delivers sustained growth
-

Park People: Zaeem Bajwa, Milton Post Office
-

Name a bus to win a delicious hamper… Mmmm!
-

Young people’s pilot scheme offers career lift off
-

Science Minister breaks ground for Nebula
-

Cancer and vaccine developments at Milton Park
-

Space to grow
-

Park People: Jay Walsh and Cody Butler, Park Club
-

Renewed Thames Travel partnership
-

Company spotlight: OBN
-

Park People: Bahija Jallal, Immunocore’s CEO
-

Didcot Powerhouse 2024 grant applications open
-

Over half of Milton Park journeys now sustainable
-
Top ten tips on using AI
-
Emergex Vaccines signs £1.79m Department of Health contract
-
Vertex announces first CRISPR gene-editing therapy
-

‘Wonder-ful’ tree planted at Milton Park on HM King’s birthday
-

Explore programme inspires nearly 1,000 Didcot students
-

Company Spotlight: Oberlanders
-

Park People: Adam from Lexica
-

Five new sustainable initiatives for Milton Park
-

Didcot Powerhouse Fund celebrates second anniversary
-
Tokamak Energy’s record-breaking machine advances commercial fusion mission
-

A whole latte love for coffee
-

Oxitec tackles disease-spreading mosquito with global partnership
-

Barnwood Limited lands Nebula contract at Milton Park
-
Innovate UK grant for Pathios Therapeutics’ cancer research collaboration
-
Isansys’ time-saving patient monitoring technology
-

Evotec opens state-of-the-art facility
-

Milton Wonder
-

Park People: Stephanie from Bright Horizons
-

Car Free Day 2023
-

Autumn’s PlantLife
-

Exscientia announces AI drug discovery collaboration with Merck
-

ThirtyFiveBio awarded £495k Innovate UK grant
-

Tokamak Energy achieves superconducting fusion magnet milestone
-

Company Spotlight: Eden Research
-

Blood pressure awareness: know your numbers
-

UK’s first full-size electric autonomous bus takes to Oxfordshire’s roads
-

Enjoy a tantalising tour of Milton Feast
-

Park People: Kathryn from MEPC Milton Park
-

Wonderful waggledancers
-

Powerhouse community pulls together to help young carers
-

Budding growth for Wild Bioscience at Milton Park
-

Honeycomb co-working space opens at the Bee House and welcomes new members
-

Park Products: aVaxziPen
-

Bee House flying high with awards success
-

Updating our amenities
-

Food app trial
-

Occupier clinical trials tackle global unmet patient needs
-

Tech award nominations for Milton Park occupiers
-

Hive Group buzzes into the Bee House
-

Park People: Saj from Convatec
-

Bee House shortlisted for South East Property Award
-

UK’s first electric autonomous bus route takes to Oxfordshire roads
-

Back to the future for Park Centre’s new name
-

Company Spotlight: Ashdown Phillips & Partners
-

Milton Park opens its doors to local students
-

Milton Park occupiers recognised as great places to work
-

Female industry leaders inspire local students into STEAM careers
-

No Mow May at Milton Park
-
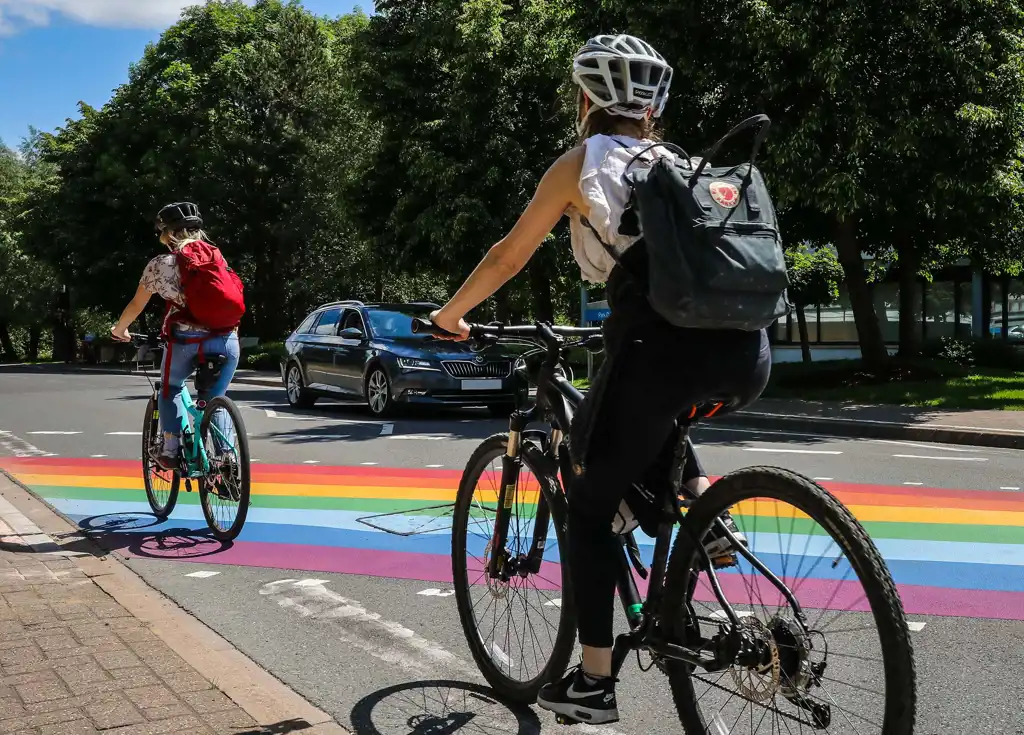
Five top tips for cycling to work at Milton Park
-

First peek at Park Centre redesign
-

Company Spotlight: Park Club
-
30 Technology sells antimicrobial platform for up to £176m
-

Park People: Katy from Whittard
-

Boost for Biotech cluster at Milton Park
-

Swiss measurement tech multi-national opens first UK R&D office at Milton Park
-

Tokamak Energy unveils fusion power plant plans
-

Explore programme launches with interactive maths panel
-

Putting a spring in your step
-

2023 Powerhouse Fund recipients announced
-

US life science company selects Milton Park as first UK manufacturing hub
-
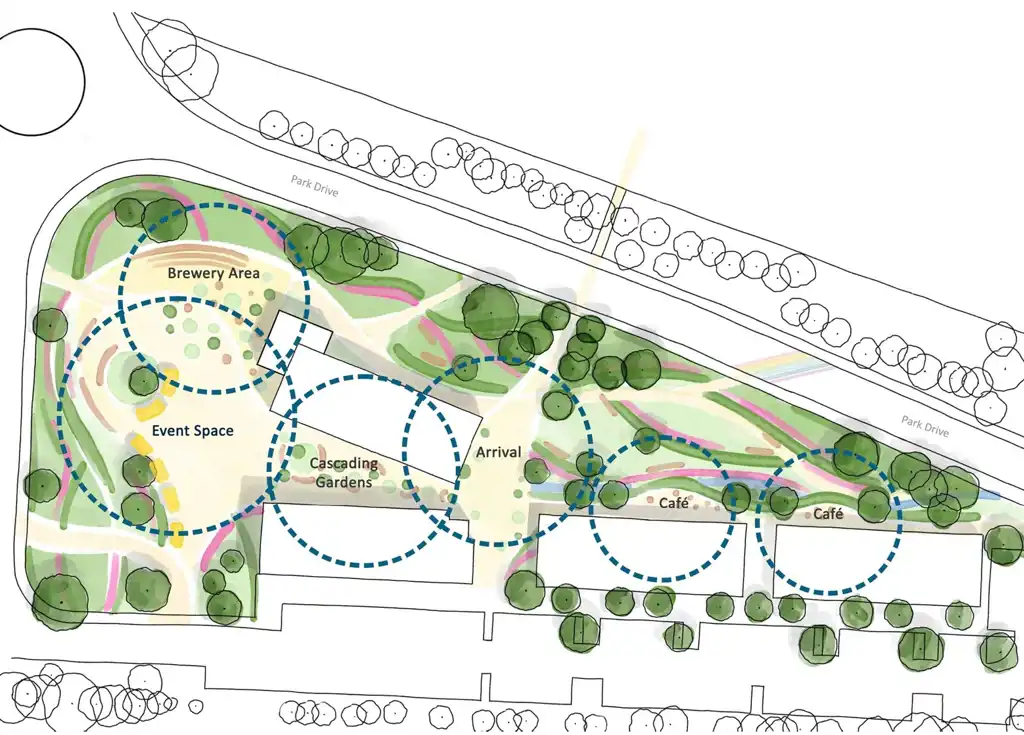
Canopies, community and connectivity: The new amenities landscape
-

Camera tech firm Living Optics snaps up Milton Park HQ
-

Park People: Charlie, Milton Park Events Coordinator
-

New 10-day simplified planning to unlock further life science and technology investment
-

Company Spotlight: Horsebox Coffee
-

Discussing the future of Oxfordshire’s innovation engine
-

Milton Park companies secure over 7% of UK’s life sciences investment
-

Exscientia to advance AI-driven platform with European collaboration
-

Park Products: Taylor & Francis industry books
-

UK’s first electric autonomous bus service begins
-

Company Spotlight: Exscientia
-

Inspiring the next generation into STEAM careers
-

Tokamak Energy appoints new Managing Director
-

Plans for new amenities are taking shape
-

Reduce your fuel bill by car sharing
-

Unlocking the potential of apprenticeships
-

Park People: Bryn Lees from Nurture Landscapes
-

Tokamak Energy builds world-first ‘super’ magnets
-

Milton Park companies leading the fight on cancer
-

Tokamak Energy awarded DOE grant to test fusion power plant materials
-

Oxfordshire buses: a greener way to travel
-

UK’s first electric autonomous bus at Milton Park
-

Park People: Milton Park Security
-

Milton Park buses: Our top five tips
-

The secrets of sustainable travel at Milton Park
-

Park People: John Chaddock from Ipsen
-

Find your nearest public EV charging point at Milton Park
-

Christmas at Milton Park
-

22 achievements in 2022
-

Christmas at Milton Park
-

We’ve won a landscaping ‘Oscar’!
-

Didcot MP launches Tokamak Energy’s first STEM outreach programme
-

Company Spotlight: Gardin
-

Meet the architects – Object Space Place
-
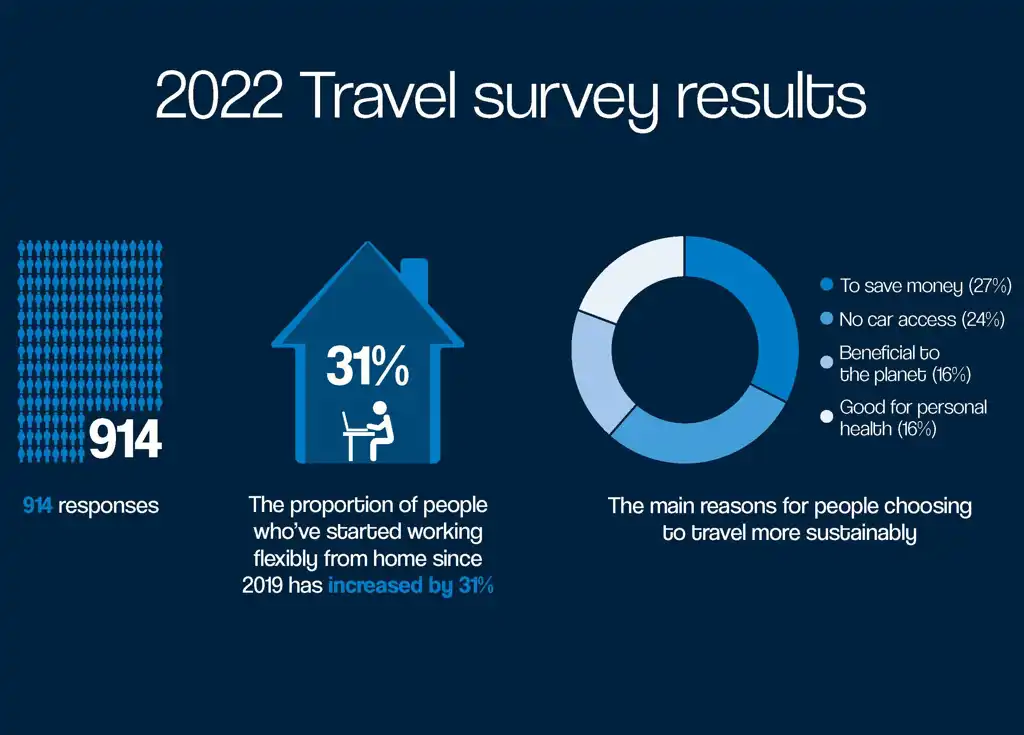
Results of the 2022 Travel Survey
-

Milton Park presents OBN’s Special Recognition Award
-

Amano Enzyme establishes new European hub at Milton Park
-

Park People: Cullen Whyte from Evotec
-

Exscientia announces US research collaboration
-
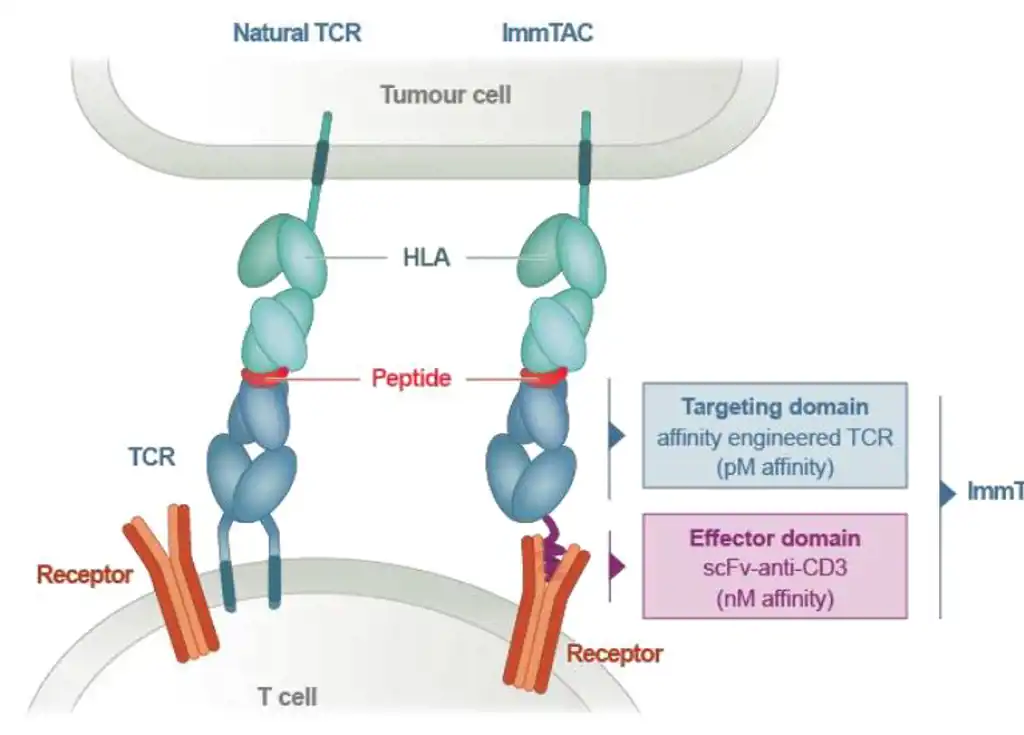
Immunocore presents new data on melanoma tumour progression
-

Milton Park to donate Christmas event money to two local charities
-

Member of Parliament visits Taylor & Francis
-

Park People: Nicole Gibbons from Hampers.com
-
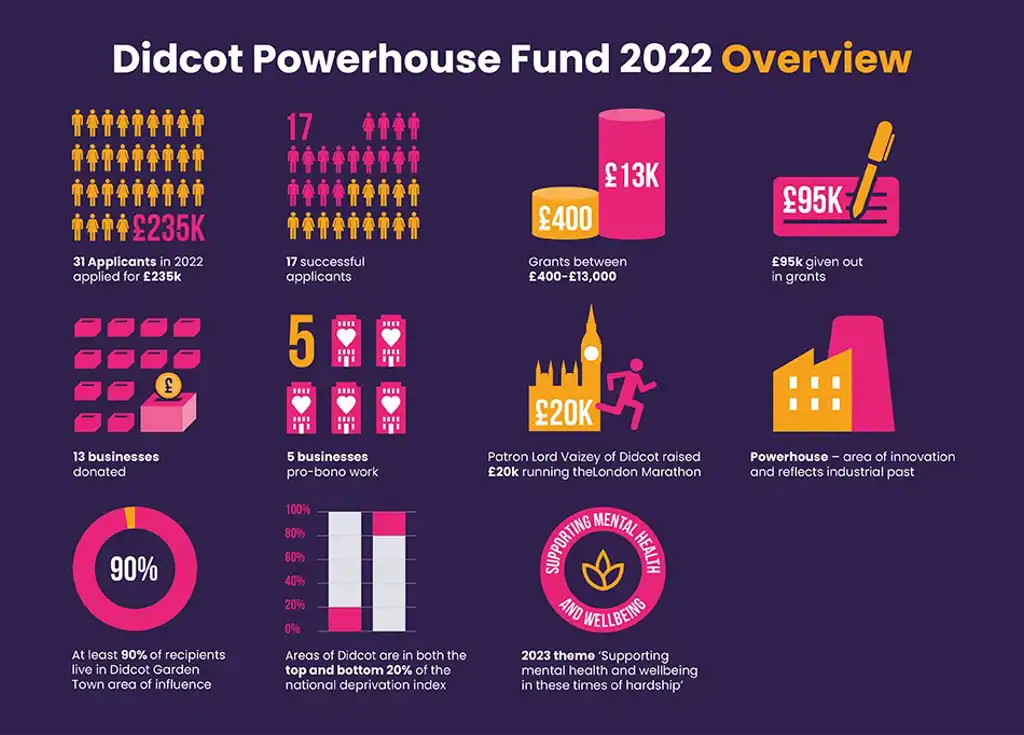
Powerhouse first anniversary
-

Keeping it square – a walkthrough of Milton Park’s what3words
-

Urban Garden Awards winners 2022
-

Second to naan fundraising
-

Love Your Liver campaign spreads awareness at Milton Park
-

Improving amenities at Milton Park
-

Eden Research sowing seeds of success
-

Company Spotlight: Vertex Pharmaceuticals
-

Park People: Vicki & Bronwynn, Park Club Personal Trainers
-

Another boost for Milton Park bus services
-

NorthRow named third most innovative at Global Software Awards
-

Evotec hosts Young Scientists Day, a STEM Outreach initiative
-

Park People: Veronica Reynolds, Sustainability & Community Manager at Milton Park
-

Oxitec expands Milton Park HQ to ramp up global fight to combat disease
-

Wildflower Wednesday
-

Microchip designer EnSilica establishes new global HQ at Milton Park
-

Next steps: Building a better future at Milton Park
-

Company Spotlight: Pathios Therapeutics
-

Green light for ‘Nebula’ R&D scheme at Milton Park
-

Park People: Dave Taylor, Nurture Landscapes
-

Green light for ‘Nebula’ R&D scheme at Milton Park
-

Park People: Mandy Nicolle, Head of Research for Biology at Biocleave
-

Taylor & Francis Holistic Hub
-

Reduce your fuel bill with the KINTO Join car share scheme
-

Have you tried the Hive Café yet?
-

A Jubilant Day at our Jubilee Street Party
-

Milton Feast
-

Park People: Mattia Poletto, R&D Programme Lead at Oxitec
-

Company Spotlight: Immunocore
-

A fond farewell to Café Metro
-

170 Brook Drive – Redevelopment
-

QUAD Systems chooses Milton Park as its first UK base
-

Didcot Powerhouse Fund awards 2022 grants
-

Spotlight: Celebrating Earth Day at Milton Park
-

Park People: Lucy Mason, Estate Services Manager at CBRE
-

New flexible workspace is the bee’s knees for sustainability
-

Milton Park – Connecting people, places and innovation
-

Over 20,000 PCR tests carried out as testing ends at Milton Park
-

Relieving stress at Milton Park
-

Fostering a climate of change at Milton Park
-

International Women’s Day – reflections from inspiring women at Milton Park
-

Park People: James Dipple, CEO of MEPC
-

Milton Park is Whittard of Chelsea’s cup of tea
-

Covid Mobile Testing Unit at Milton Park
-

Park People: A Day in the life of Farah Nazir-Chapman, Planet IT
-

Company Spotlight: Nexeon
-

Keep your eyes peeled for our new orange bikes!
-

Helping apprentices #BuildTheFuture at Milton Park
-

Evotec to undergo sustainable expansion at Milton Park
-

The Didcot Powerhouse Fund Opens for Grants Applications
-
Park People: A Day in the life of Hannah Willett, Plasma Diagnostician at Tokamak Energy
-

Updated bus timetable for 2022
-
Company Spotlight: Emergex Vaccines
-

Park People: A Day in the life of Suzanne Harvey, Operations Manager at Ipsen
-

Isansys win big at OBN Awards
-

Another great year for our wreath-making workshops
-

Urban Garden Awards Winners!
-

Vertex inspiring future scientists in Learning Lab at Milton Park
-

Getting involved in the global conversation: Tokamak Energy, Milton Park and COP26
-

Shining a light on businesses at Milton Park
-

Introducing the Didcot Powerhouse Fund – Fuelling Better Futures
-

Park People: A day in the life of Georgina Horton, Isansys Lifecare
-

Thinking of cycling to work? It’s easy!
-

Park People: A Day in the life of Philip Campbell, Commercial Director
-

What will your three words be?
-

Join us for One Great Day
-

Milton Park occupiers help the Government create a vision for the future
-

We’re BALI Award winners!
-

Sneak peek at our Hive Café
-

Outdoor games at Milton Park
-

Urban Garden Awards
-

Exscientia selects Milton Park for new laboratories
-

Recharge. Refresh. Reconnect
-

Tokamak Energy signals major expansion
-

Geese Islands
-

New free bike rental hub open at Didcot Parkway
-

More sustainable ways to travel to Milton Park
-

Helping to save the bee at Milton Park
-

Milton Park security team’s new electric cars
-

Business Secretary visits Johnson Matthey at Milton Park to open new battery technology facility
-

Greener Workplace Forum launches at Milton Park
-

Vale of White Horse District Council and MEPC to review Milton Park Local Development Order to meet 2040 Vision and respond to climate emergency
-

Thames Travel unveils new Milton Park buses livery as part of boost to sustainable transport connectivity
-

Emergex Vaccines expands at Milton Park
-

Choose your company’s what3words square
-

New initiative to stop plastic waste reaching waterways
-

Develop your public speaking skills in a supportive environment
-

Evotec expands again at Milton Park
-

Milton Park installs new cycle and foot bridge linking to Abingdon and Oxford
-

New M&E contractor appointed
-

Milton Park adds touch-free sensors to new pedestrian crossings
-

Milton Park honey winners announced
-

Have you seen our what3words campaign?
-

Take a look at our new community offers and discounts
-

2020 year in review in numbers
-

Watch: make your own Christmas wreath thanks to Nurture
-

Milton Park refreshes brand identity for a changing world and 2040 Vision
-

Don’t forget we have Amazon lockers at Milton Park
-

Check out our new rainbow and levitating pedestrian crossings
-

New defibrillator for nursery thanks to fundraising
-

Evotec expands at Milton Park to become one of its largest occupiers
-

Top tips for commuting to work by bike
-
Have you signed-up to Mi-Link, your easy travelling to work planner?
-

MEPC welcomes ten new science and tech companies to Milton Park
-

MEPC welcomes ten new science and tech companies to Milton Park
-

A round-up of companies at Milton Park helping with important COVID-19 response
-

Amazing Scientific Ideas from Oxfordshire
